Grass Spikelet Structures of Diagnostic Value
Deborah J. Lionakis Meyer
23 February 2022
The grass spikelet is often the dispersal unit, disseminule, or seed unit for the grass plant. Grass flowers lack the colorful, showy parts that would normally spark human curiosity towards identifying a plant. The structures associated with a grass flower are usually bland shades of green, yellow, or brown. Many people shy away from studying grasses because of their perceived difficulty to identify. In the grass family there is considerable variation in the appearance of the structures surrounding the grass flower that can lead to confusion when attempting to make an identification; however, the ability to recognize and understand the placement of the structures within the spikelet can make identification at, subfamily, tribe, genus, and species level much easier. Here the discussion will focus on the basic diagnostic features of the grass spikelet and their locations.
The grass flower is simple in structure, typically consisting of a pistil, three stamens, and two lodicules (Figure 1). The pistil consists of an ovary, containing a single ovule, to which two styles are attached at the apex, each supporting a feathery stigma. Grasses are wind pollinated and the feathery stigmas provide ample surface area for pollen grains to attach. The long, thread-like filaments of the stamens are attached at the base of the flower. At the distal end of the filament is attached the pollen-producing anther. The tiny, hyaline, scale-like lodicules are located at the base of the flower and are considered to be representative of the perianth found in other flowering plants; grasses lack the showy petals and sepals found in many other flowering plants. The lodicules swell during anthesis, forcing open structures surrounding the flower and exposing the stigmas for pollination. The parts of the grass flower that may be useful to identification of mature dispersal units may include the length and colour of the anthers (if present), the remnants of the style bases that may remain attached to the apex of the mature fruit, and the shape of the lodicules.
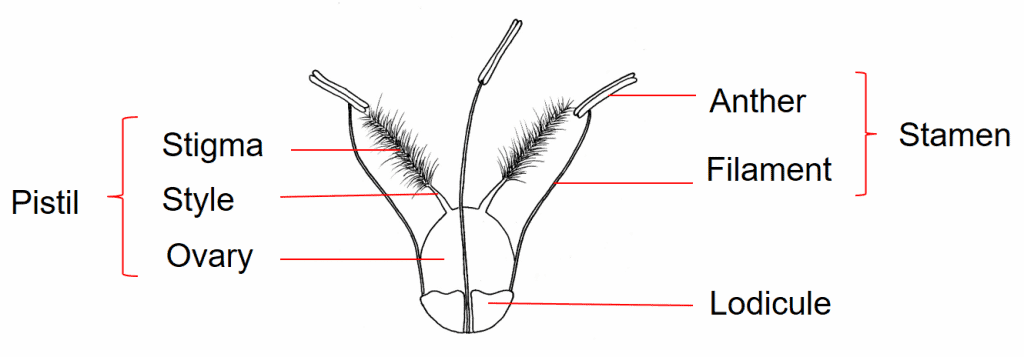
Figure 1. Typical grass flower consisting of one pistil with a superior ovary, three stamens, and two lodicules. From Meyer, 2001.
The one-seeded indehiscent fruit of a grass plant is called a caryopsis (grain) and it is derived from the superior ovary. The ovule is attached to the placenta of the ovary wall by a short funiculus (stalk) along the ventral suture of the ovary or at the ovary base. This point of attachment is visible as either a line or a spot on the ventral surface of the caryopsis and in grasses this area is called the hilum (Figure 2).

Figure 2. Grass caryopses in ventral view showing different shapes of the grass hilum, in which the point of attachment of the mature seed (former ovule) within the fruit is attached by a short funiculus to the pericarp (former ovary wall). From left to right, Eragrostis curvula (weeping lovegrass), Cenchrus echinatus (southern sandbur), and Deschampsia danthonioides (annual hair grass), in which the hilum is a small spot near the base of the caryopsis and Lolium perenne (perennial ryegrass) and Elymus repens (couchgrass or quackgrass) in which the hilum is long and linear. (H = hilum)
The outline of the embryo can be seen on the dorsal side of the caryopsis (Figure 3).

Figure 3. Grass caryopses in dorsal view showing different shapes and sizes of the embryo compared to the length of the caryopsis. The red line indicates the upper end of the embryo. In these examples, the ratio of embryo to caryopsis length ranges from about one-quarter the length of the caryopsis to equal to the length of the caryopsis. From left to right: Ventenata dubia (soft ventgrass), Deschampsia cespitosa (tufted hairgrass), Diplachne fusca subsp. fascicularis (bearded sprangletop), Setaria italica (foxtail millet), Cenchrus spinifex (coast sandbur), Zizania aquatica (annual wildrice).
The embryonic root-shoot axis is attached to the single, shield-shaped, cotyledon called the scutellum, which is pressed up against the endosperm and is modified to absorb nutrients from the endosperm during seed germination and seedling development (Figure 4).
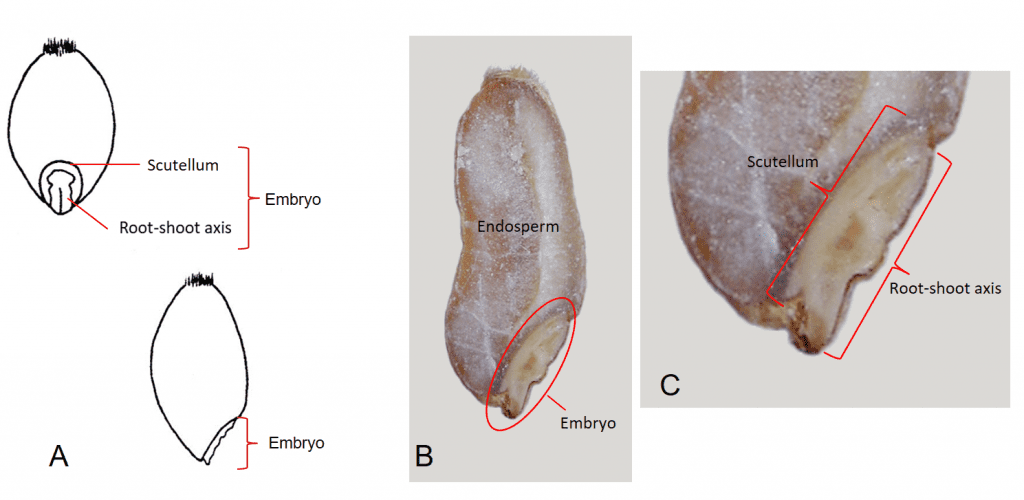
Figure 4. Triticum aestivum (common wheat) caryopsis. A – Dorsal view (top) and lateral view (bottom) of caryopsis. B – Longitudinal section of caryopsis exposing the embryo in lower right area of caryopsis and remaining portion of the caryopsis filled with solid endosperm. C – Close-up view of embryo showing the root-shoot axis immediately beneath the outer dorsal surface of the pericarp and the scutellum (modified cotyledon) compressed between the root-shoot axis and the endosperm.
Mature caryopses may be nearly round in cross-section or compressed (Figure 5).
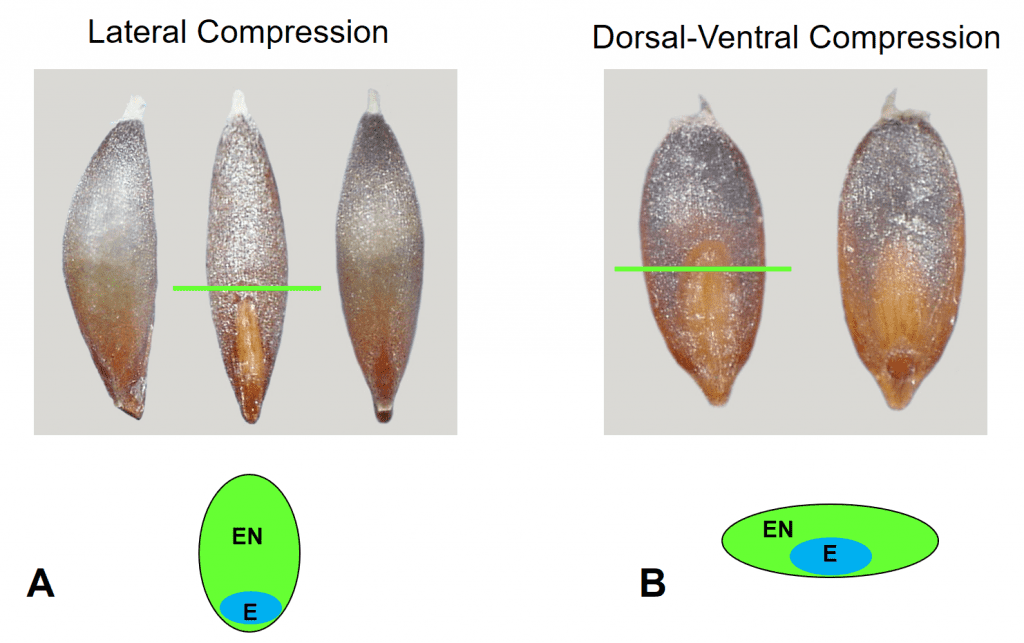
Figure 5. Grass caryopsis compression. A – Lateral compression of caryopsis in Deschampsia danthonioides (annual hair grass). Caryopsis shown from left to right in lateral, dorsal, and ventral views, and the cross-sectional view illustrated below. B – Dorsal-ventral compression of caryopsis in Diplachne fusca subsp. fascicularis (sprangletop). Caryopsis shown from left to right in dorsal and ventral views, and the cross-sectional view illustrated below. The green line indicates the point of cross-sectional slice. (E = embryo, EN = endosperm).
The shape and length of the hilum, length of the embryo relative to length of the caryopsis, and compression of the caryopsis can be useful diagnostic features (Figure 6). Typically, the bulk of the mature caryopsis is filled with endosperm (Figure 4). During early development the endosperm is somewhat fluid in composition and as the caryopsis matures the endosperm may remain fluid or become semi-solid to solid depending on the species. In a study of mature caryopses of 169 grass genera, Terrell (1971) categorized the condition of endosperm as liquid to solid (Table 1). Liquid endosperm in mature caryopses can remain in the liquid state for up to 50 years (Dore, 1956; Terrell, 1971). The condition of endosperm in the mature caryopsis is a useful diagnostic character.
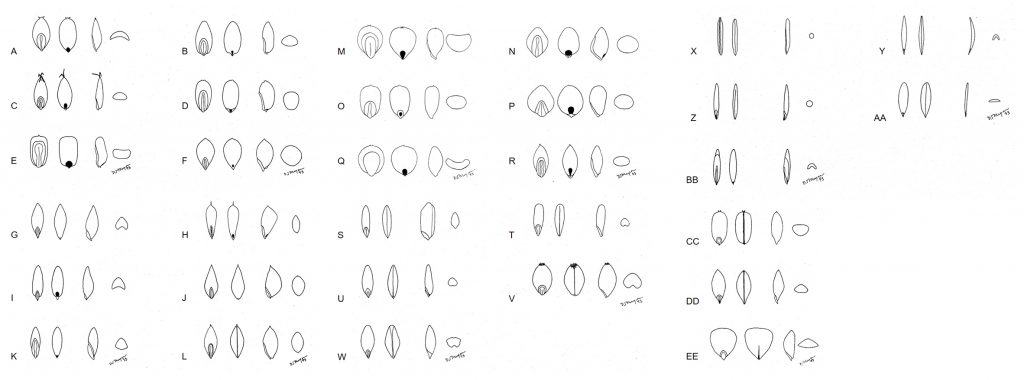
Figure 6. Grass caryopses in dorsal view showing embryo, ventral view showing hilum, lateral view, and cross-sectional view (dorsal side up). A – Diskisperma dubium, green sprangletop; B – Eragrostis curvula, weeping lovegrass; C – Digitaria sanguinalis, crabgrass; D – Sporobolus airoides, alkali sacaton; E – Cenchrus ciliaris, hairy buffelgrass; F – Phleum pratense, timothy; G – Poa trivialis, rough bluegrass; H – Alopecurus arundinaceus, creeping foxtail; I – Dactylis glomerata, orchardgrass; J – Phalaris minor, littleseed canarygrass; K – Chloris gayana, rhodesgrass; L – Phalaris canariensis, canarygrass; M – Cenchrus americanus, pearl millet; N – Echinochloa crus-galli, barnyardgrass; O – Cenchrus clandestinus, kikuyugrass; P – Panicum miliaceum, proso millet; Q – Sorghum bicolor, sorghum; R – Panicum virgatum, switchgrass; S – Oryza sativa, rice; T – Secale cereale, rye; U – Avena sativa, oat; V – Triticum aestivum, common wheat; W – Hordeum vulgare, barley; X – Zizania aquatica, wild rice; Y – Bromus diandrus var. rigidus, ripgut brome; Z – Nassella cernua, nodding stipa; AA – Bromus inermis, smooth brome; BB – Bouteloua eriopoda, black grama; CC – Glyceria declinata, waxy mannagrass; DD – Melica californica, California melic; EE – Briza maxima, big quakinggrass.
Table 1. Examples of endosperm condition in mature caryopses. Determination made by pressing on caryopses with sharp-pointed forceps (Terrell, 1971). Table from Meyer (2020).
|
Endosperm state |
Description |
Example genera |
|
Liquid |
Flows readily as a thick liquid | Koeleria, Trisetum, Agrostis |
|
Semi-liquid |
Flows only slightly | Agrostis, Alopecurus, Briza |
|
Soft |
Does not flow but appears moist and is easily crushed | Agrostis, Alopecurus, Briza, Dactylis, Holcus |
|
Semi-solid |
Crushed with small amount of pressure and appears dry | Agrostis, Arrhenatherum, Avena, Briza |
|
Solid |
Does not yield to moderate or strong pressure | Agropyron, Bromus, Festuca, Hordeum, Oryza. Panicum, Poa, Triticum, Zea |
With few exceptions, the pericarp closely adheres to the seed coat (testa) surface, and most of the cell layers of the pericarp and seed coat are crushed together during the growth of the embryo and endosperm within the seed.
An example of a caryopsis with a loose-fitting pericarp can be found in Eleusine indica (goosegrass) in which the pericarp is papery thin and can be easily peeled away to reveal the true seed that has a highly sculptured seed coat surface (Figure 7).

Figure 7. Eleusine indica (goosegrass) florets, caryopses with hyaline (thin, whitish, and translucent) pericarps, and true seeds with pericarp removed (inset).
A grass flower, and later the caryopsis, is usually enclosed by two bracts to form the fertile floret. The upper bract is called the palea and the lower bract is called the lemma. In the mature floret the palea conceals the hilum of the caryopsis and the lemma conceals the embryo. Like the caryopsis, the floret may not be compressed (round in cross-section) or may be compressed dorsal-ventrally or laterally (Figure 8). In addition to the fertile floret, some species may also produce florets containing only stamens (staminate floret) or only a pistil (pistillate floret) or florets lacking reproductive structures or a caryopsis (sterile floret) or simply an empty lemma (sterile lemma).
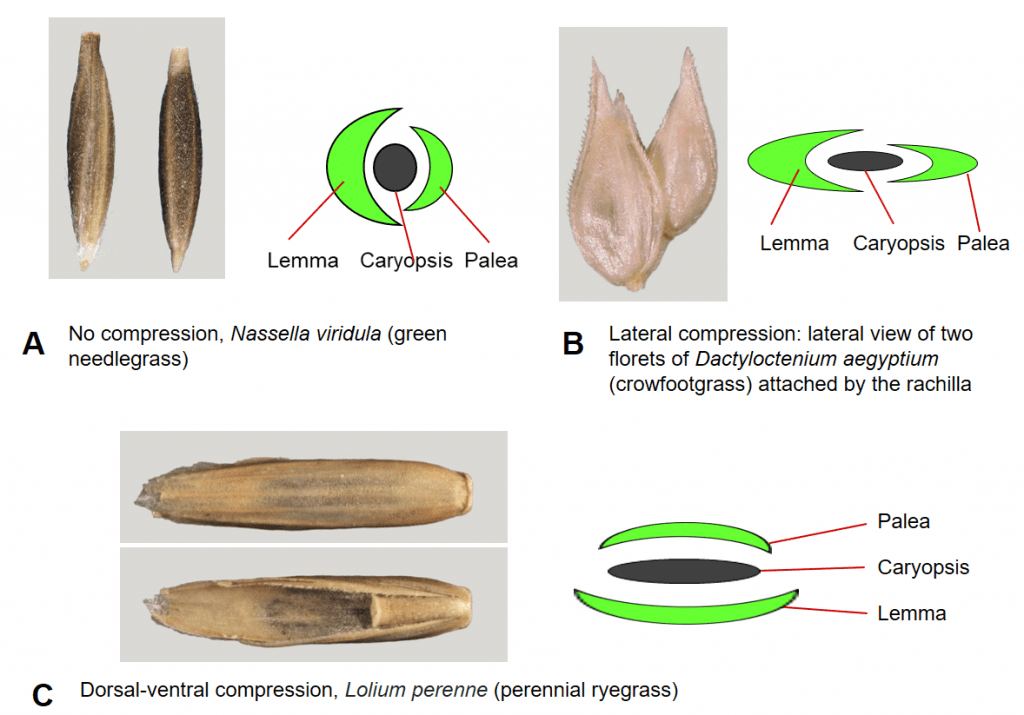
Figure 8. Floret compression types. A – No compression, in which the lemma and palea encircle a nearly round caryopsis. B – Lateral compression, in which the lemma and palea are folded inward longitudinally and the two sides are compressed together. C – Dorsal-ventral compression, in which the lemma on the dorsal side of the floret (top) is compressed nearly flat against the embryo side of the caryopsis and the palea on the ventral side of the floret is pressed nearly flat against the hilum side of the caryopsis (bottom).
The following features of the lemma and palea can be valuable diagnostic characters:
- Length of the lemma and palea, individually and relative to each other (Figure 9.)
- Length of the lemma or palea relative to the length of the caryopsis
- Number of longitudinal nerves, the lemma typically has an odd number of nerves and the palea usually has an even number.
- Presence, length, location, and characteristics of the awn (e.g., straight, bent, twisted) (Figure 10).
- Presence or absence of a lemma keel (Figure 11).
- Presence, shape, and length of lemma teeth (Figure 12).
- The shape, size and surface texture of the lemma callus (Figure 13).
- Thickness (membranous, papery, hardened), sheen (dull, lustrous, glaucous, shiny, glossy), and surface texture (see surface feature comparison chart) (Figure 14).
- Presence of hairs along the palea keels and their length and spacing (Figure 15).
- Presence or absence of surface hairs on the lemma and/or palea and their types and locations (Figure 16).

Figure 9. Relative length of palea to lemma can range from palea absent, shorter than the lemma, to equal to the lemma in length. Examples from left to right include, Agrostis capillaris (colonial bentgrass) with palea about one-half the length of the lemma, Bromus hordeaceus (soft chess), Diplachne fusca subsp. fascicularis (bearded sprangletop), Deschampsia cespitosa (tufted hair grass) with palea progressively longer compared to the lemma length, and Diplachne fusca subsp. uninervia (Mexican sprangletop) with palea nearly equal in length to the lemma.
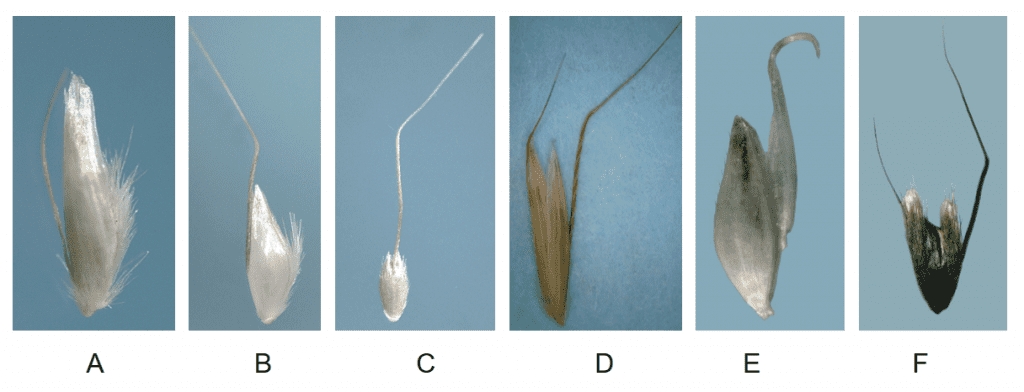
Figure 10. Examples of lemma awn positions on fertile and sterile florets: A – fertile floret with basal awn in Deschampsia cespitosa (tufted hairgrass); B – fertile floret with awn from mid-point of lemma in Danthonia danthonioides (annual hairgrass); C – fertile floret with awn from near tip of lemma in Gastridium ventricosum (nit grass); D – both fertile and sterile florets with bent awns in Arrhenatherum elatius (bulbous oatgrass); E – fertile floret lacking an awn and sterile floret with hooked awn in Holcus lanatus (velvetgrass); F – fertile floret lacking an awn and two sterile florets with different types of awns in Anthoxanthum odoratum (sweet vernalgrass).
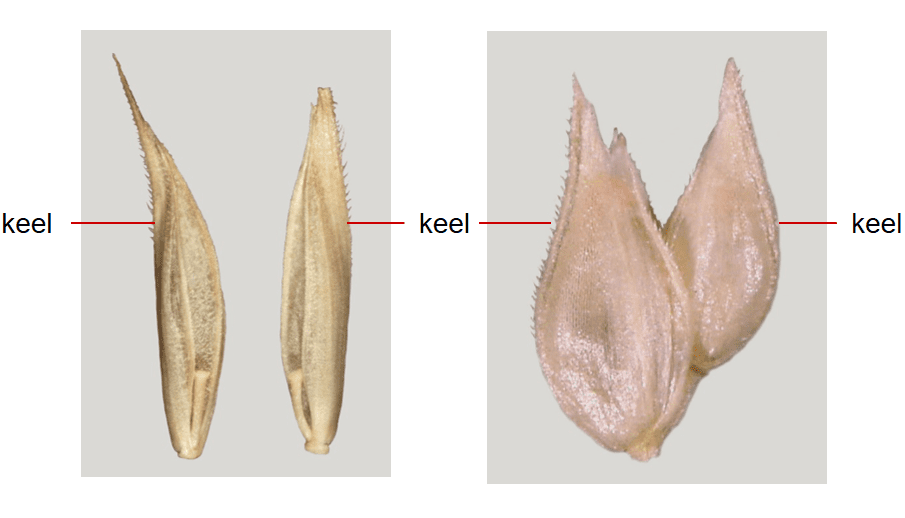
Figure 11. Lemma with keel or longitudinal ridge along the center of the folded lemma in a laterally compressed floret. A – Dactylis glomerata (orchardgrass). B – Dactyloctenium aegyptium (crowfoot grass).
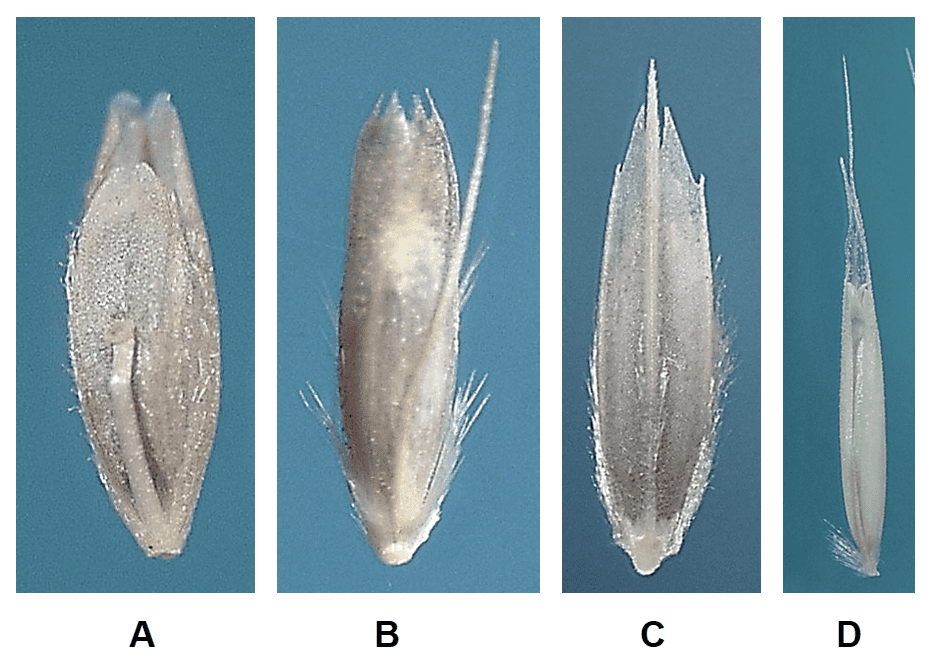
Figure 12. Examples of lemma teeth: A – lobed teeth at apex of lemma in Dinebra panicea subsp. panicea (thread sprangletop); B – Deschampsia cespitosa (tufted hairgrass) with four pointed teeth at the lemma apex; C – Diplachne fusca subsp. fascicularis (bearded sprangletop) with two pointed teeth at lemma apex flanking the awn; D – Ventenata dubia (soft ventgrass) with two awn-like lemma teeth at lemma apex flanking the awn.
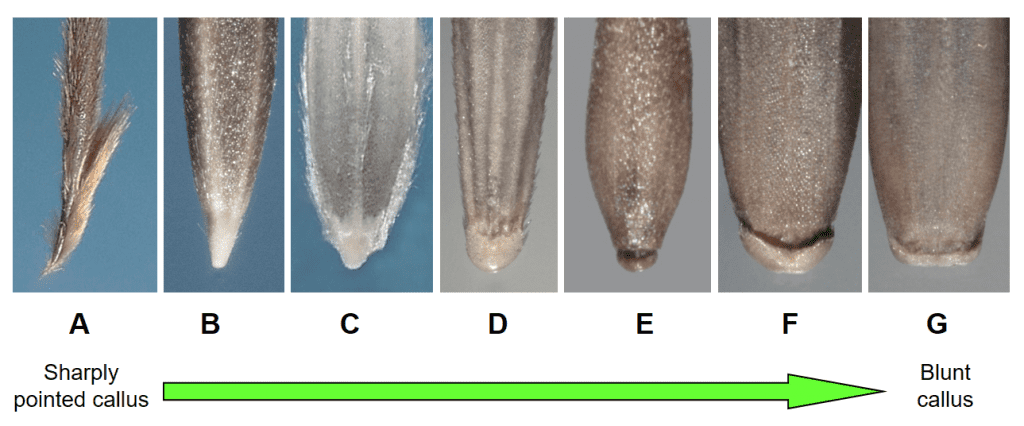
Figure 13. Examples of lemma callus shapes: A – sharply pointed, Heteropogon contortus (tanglehead); B – long and pointed, Nassella viridula (green needlegrass); C – short and pointed, Diplachne fusca subsp. fascicularis (bearded sprangletop); D – thick and slightly pointed, Bromus sterilis (barren brome); E – narrow and rounded to blunt, Apera spica-venti (windgrass); F – thick and nearly blunt with indentation at mid-point, Festuca arundinacea (tall fescue); and G – narrow and blunt, Lolium perenne (perennial ryegrass).

Figure 14. Examples of lemma and palea structural thickness and sheen: A – hyaline and lustrous lemma of Agrostis capillaris (colonial bentgrass); B – papery lemma and membranous palea in Bromus hordeaceus (soft chess); C – papery lemma and palea in Festuca arundinacea (tall fescue); D – leathery and shiny lemma and palea in Phalaris paradoxa (hooded canarygrass); E – leathery to shell-like and lustrous lemma and palea in Nassella viridula (green needlegrass); F – shell-like and dull lemma and palea in Oryza sativa (rice); G – shell-like and glossy lemma in Panicum miliaceum subsp. ruderale (wild proso millet).
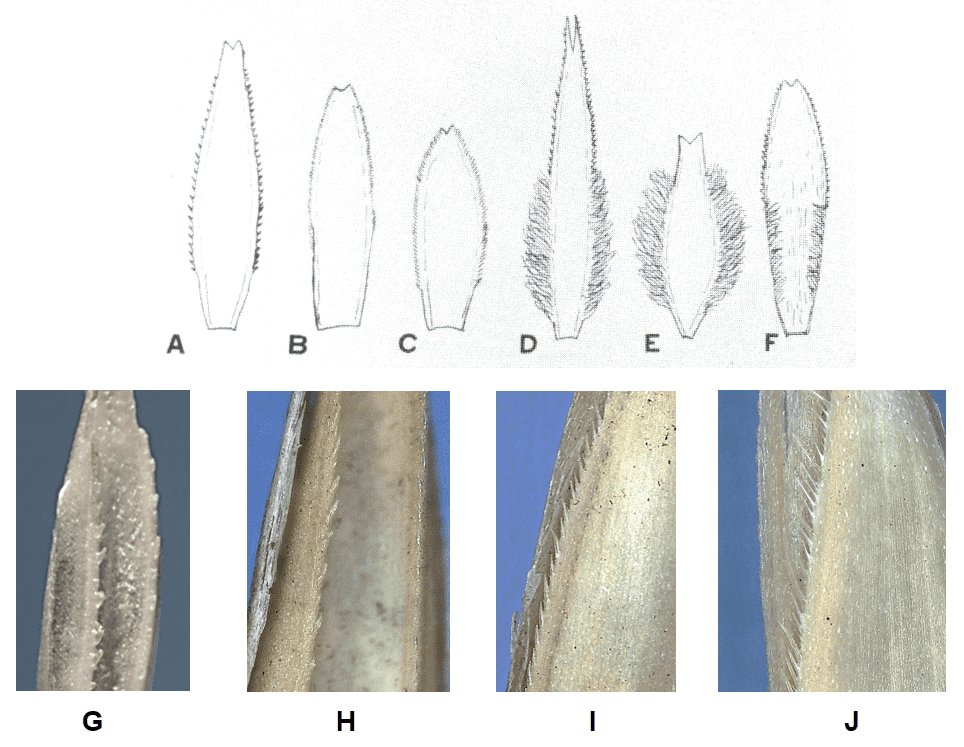
Figure 15. Examples of hair length and spacing on the palea keels (also referred to as palea teeth): A – Poa pratensis (Kentucky bluegrass); B – P. trivialis (rough bluegrass); C – P. compressa (Canada bluegrass); D – P. arachnifera (Texas bluegrass); E – P. annua (annual bluegrass); F – P. arida (plains bluegrass); G – Agropyron desertorum (standard crested wheatgrass); H – Elymus repens (quackgrass, couch grass); I – Elymus trachycaulus (slender wheatgrass); J – Pascopyrum smithii (western wheatgrass). Illustrations in A – F are from Musil (1963).
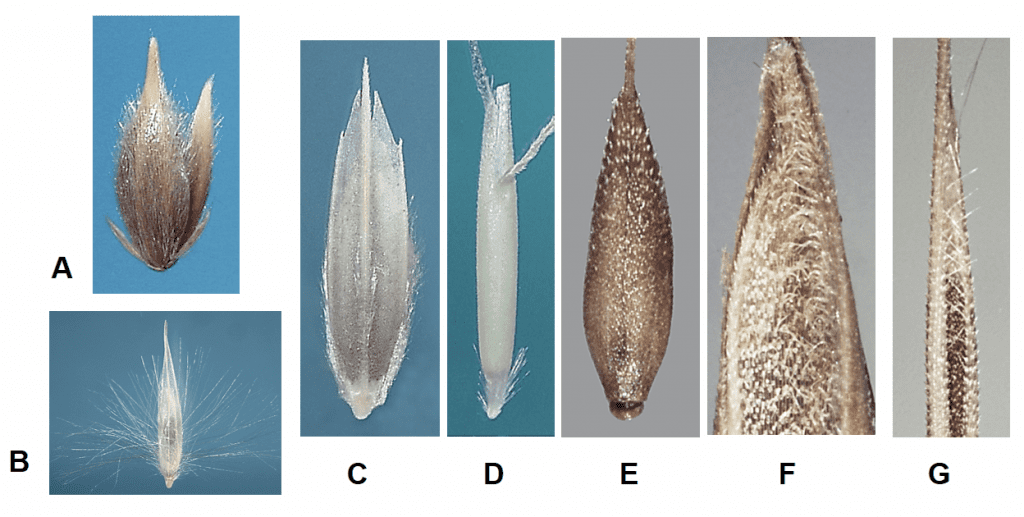
Figure 16. Examples of hair placement on the lemma and palea: A – short silky hairs covering the lemma and palea in Phalaris lemmonii (Lemmon’s canarygrass); B – long silky hairs on the lower half of the lemma in Arundo donax; C – hairs along the lower portion of lemma nerves and across upper edge of the callus in Diplachne fusca subsp. fascicularis (bearded sprangletop); D – short silky hairs covering lemma callus in Ventenata dubia (soft ventgrass); E – short stiff hairs in upper half of lemma in Apera spica-venti (windgrass); F – hairs at the tip of the palea in Festuca rubra (red fescue); G – long hairs along the upper margins of the lemma in Vulpia myuros (annual fescue).
Depending on the species, a grass spikelet can contain one or more florets subtended by one or typically two glumes. The florets within a spikelet may all have the same appearance or may appear quite different in size and shape. Some spikelets may contain all fertile florets while other spikelets may contain a mixture of fertile florets and sterile or staminate florets or may include sterile lemmas. Grass dispersal units disarticulate from the inflorescence in a variety of ways. The disarticulation from the plant can occur below the glumes, in which case the glumes become part of the dispersal unit, or above the glumes, in which case the glumes remain on the plant and the florets become the dispersal units. Occasionally the lemma and/or palea remain attached to the plant and the caryopsis breaks free as the dispersal unit. The central axis of the spikelet to which the florets are attached is called the rachilla. When the florets break apart from each other at the nodes of the rachilla a portion of the rachilla may remain attached to the palea side of the floret (Figure 17).
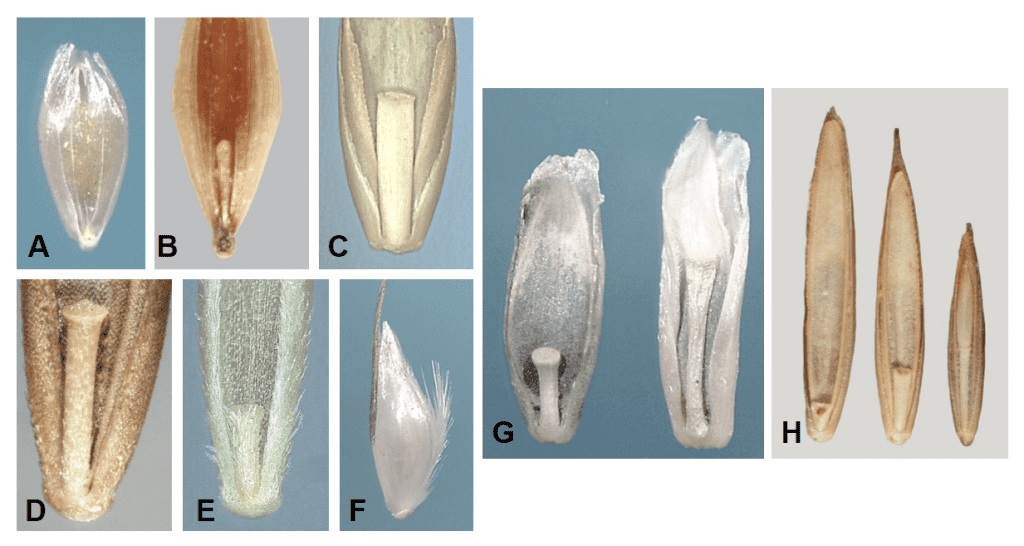
Figure 17. Examples of rachilla segments: A – the floret of Polypogon monspeliensis (rabbit foot grass) from the single floret spikelet lacks a rachilla; B – Holcus lanatus (velvetgrass) has a thin, wire-like rachilla; C – Lolium temulentum (darnel) has a smooth, strap-like rachilla; D – Festuca arundinacea (tall fescue) has a round, rod-like rachilla that flares at the apex; E – Festuca octoflora (six-weeks fescue) has rod-shaped rachilla covered with hairs; F – Deschampsia danthonioides (annual hairgrass) has a thin wire-like rachilla covered with long silky hairs (shown in lateral view); G – Disakisperma dubium (green sprangletop) rachilla length tends to be shorter in the lower florets and longer in the distal fertile floret of the spikelet (right); H – Elymus repens (quackgrass or couch grass) rachilla shape and length differs depending on position of the floret in the spikelet (left floret from basal position, middle floret from middle position, and right floret from distal position within the spikelet).
Dispersal units of grasses can include single florets, groups of florets, spikelets, and clusters of spikelets, some of which may have attached accessory structures (Figure 18).
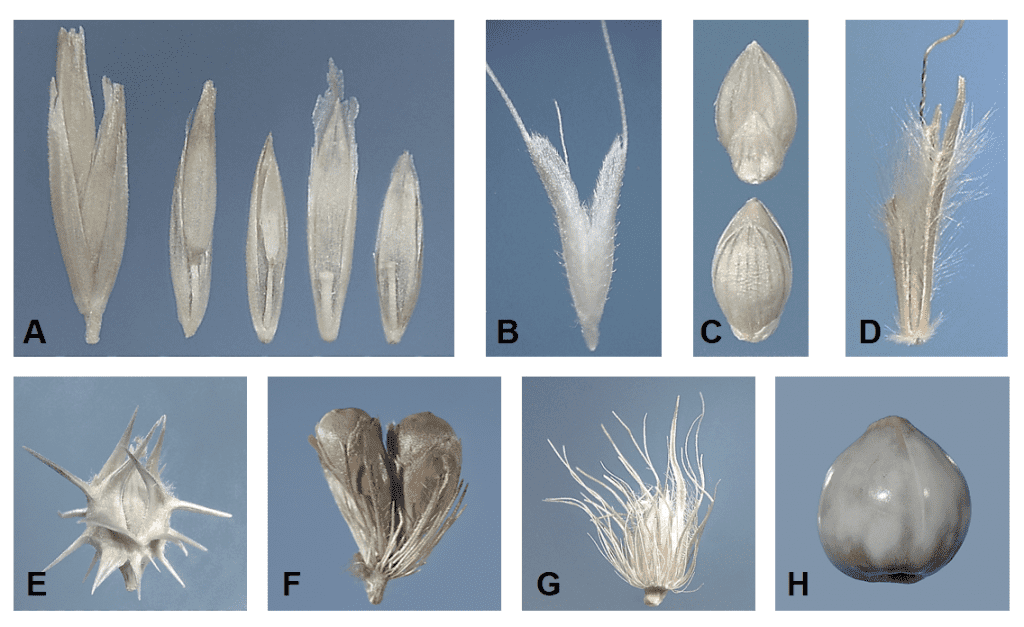
Figure 18. Types of dispersal unit disarticulation. Disarticulation above the glumes and between the florets along the rachilla (central axis of the spikelet): A – in Poa secunda (big bluegrass). Disarticulation below the glumes and the glumes are part of the dispersal unit: B – Polypogon monspeliensis (rabbit foot grass) and C – Setaria faberi (giant foxtail). Disarticulation below the glumes and each dispersal unit comprised of a spikelet with attached rame (rachis) segment and pedicel with attached pedicellate spikelet: D – Schizachyrium scoparium (little bluestem). Disarticulation below two whorls of fused bristles forming a bur-like fascicle enclosing two to four spikelets: E – Cenchrus spinifex (coast sandbur). Disarticulation below whorls of bristles forming a fascicle surrounding one to several spikelets: F – Cenchrus americanus (pearl millet) and G – Cenchrus ciliaris (buffelgrass). Disarticulation below involucre enclosing the pistillate spikelet: H – Coix lacryma-jobi (Job’s-tears).
Occasionally, vegetative reproductive structures called bulbils will form instead of a caryopsis filled floret within the spikelet (Figure 19).
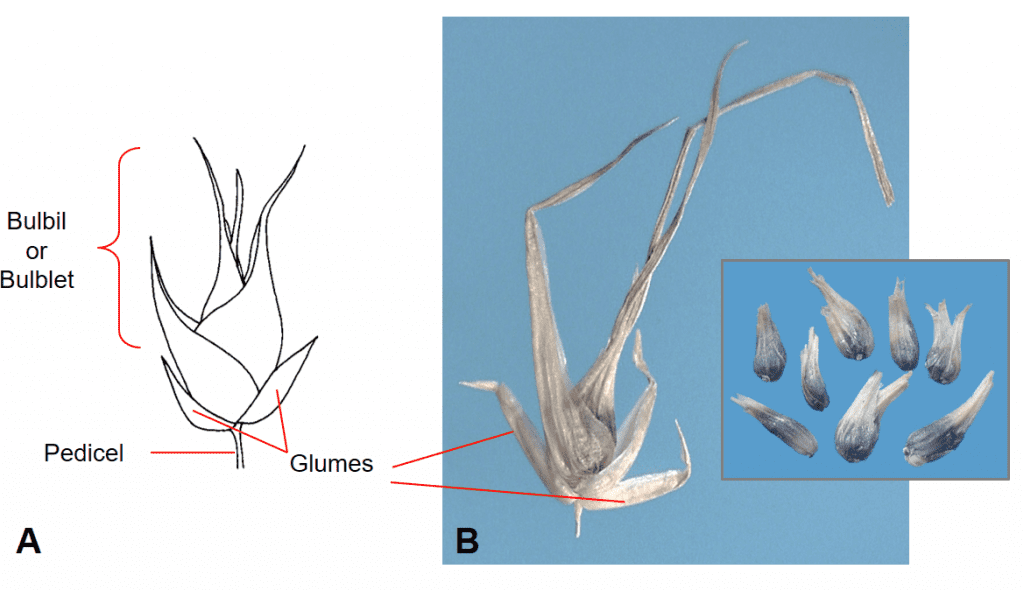
Figure 19. Poa bulbosa subsp. vivipara (bulbous bluegrass). An aerial bulbil (bulblet) subtended by two glumes replaces the typical floret filled spikelet, as found in P. bulbosa subsp. bulbosa, and serves as the dispersal unit for vegetative reproduction. A – Diagram of bulbil (bulblet) in spikelet. B – Spikelet with bulbil removed from plant showing the length of the leaves that comprise to bulbil and milled bulblets found as contaminants in a seed sample (inset).
The following features of the spikelet can be valuable diagnostic characters:
- Type of dispersal unit disarticulation (Figure 18).
- Number of glumes (Figure 20).
- Length of the glumes relative to each other and to the length of the entire spikelet (Figure 20).
- Number of nerves on each glume.
- Presence or absence, location and length of awns on the glumes (Figure 21).
- Structural thickness (membranous, hyaline, papery, leathery, shell-like), sheen (dull, lustrous, glaucous, shiny, glossy), and surface texture (see surface feature comparison chart) of glumes (Figure 22).
- Dorsal-ventral or lateral compression of the spikelet (Figure 23), floret (Figure 8), and caryopsis (Figure 5).
- Number of fertile florets and number and location of sterile or staminate florets or sterile lemmas within a spikelet, if present (Figure 24 – 42).
- Length, width, shape, and texture of the rachilla, if present (Figure 17).
- Presence or absence of accessory structures such as pedicel segment, rachis segment, rame segment, subtending spiny bracts and bristles forming burs and fascicles, and subtending and enclosing involucre (Figure 18).
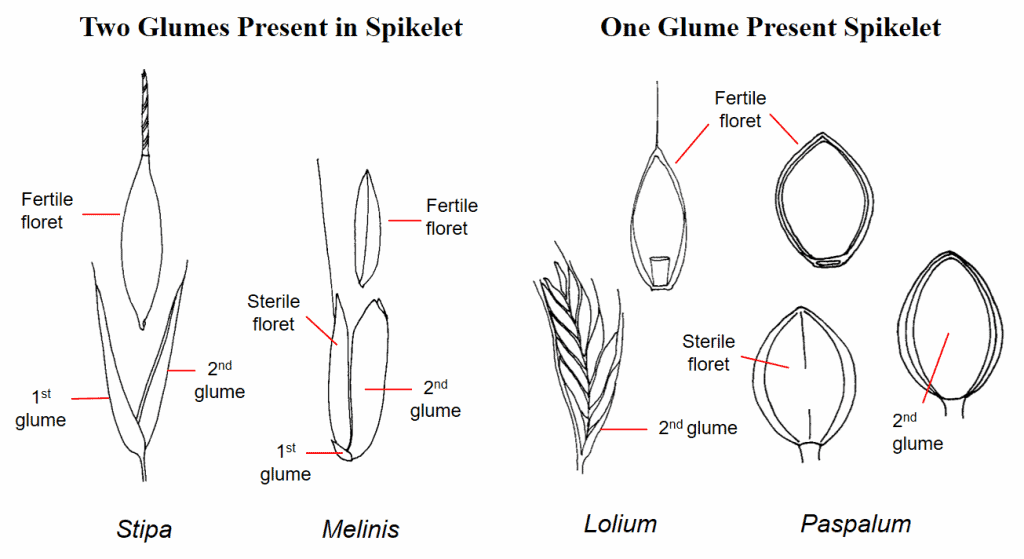
Figure 20. Examples of numbers of glumes present in a spikelet. In Stipa (needlegrass) the first and second glume are of nearly equal length. In Melinis (molassesgrass) the first glume is very small, and the second glume is equal to the length of the whole spikelet. Lolium (ryegrass) spikelets lack a first glume, and the second glume is usually shorter than the whole spikelet. The Paspalum (paspalum) spikelet lacks a first glume, and the second glume is nearly the same length as the spikelet.
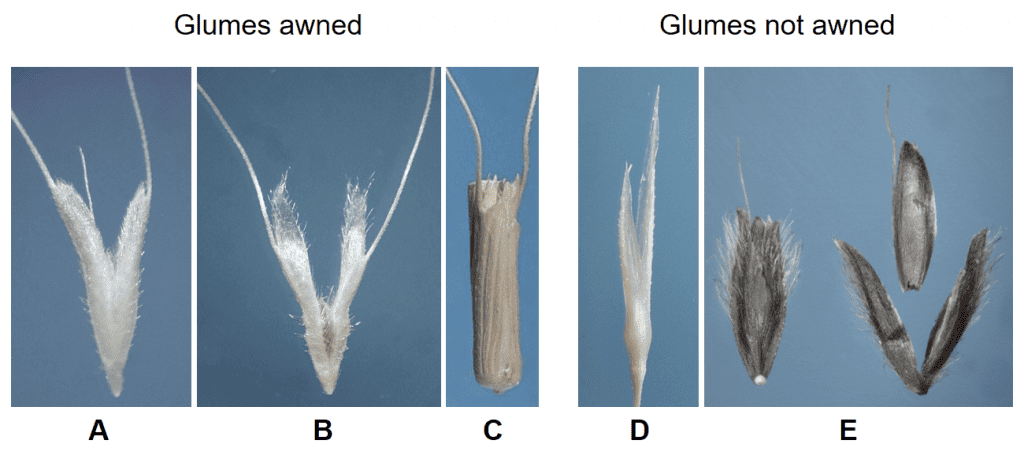
Figure 21. Examples of glumes with and without awns in natural grass dispersal units: A – Polypogon monspeliensis (rabbit foot grass) both glumes are awned near the apex (fertile floret held within the glumes is also awned); B – Polypogon maritimus (Mediterranean beardgrass) both glumes are awned in upper third of the glume; C – Aegilops cylindrica (jointed goatgrass) the glumes awned at their apex; D – Gastridium ventricosum (nit grass) glumes are long and tapered but not awned; E – Alopecurus arundinaceus (creeping foxtail) intact spikelet (left) and dissected spikelet (right) demonstrate the floret lemma is awned, but the glumes of the spikelet are not awned.
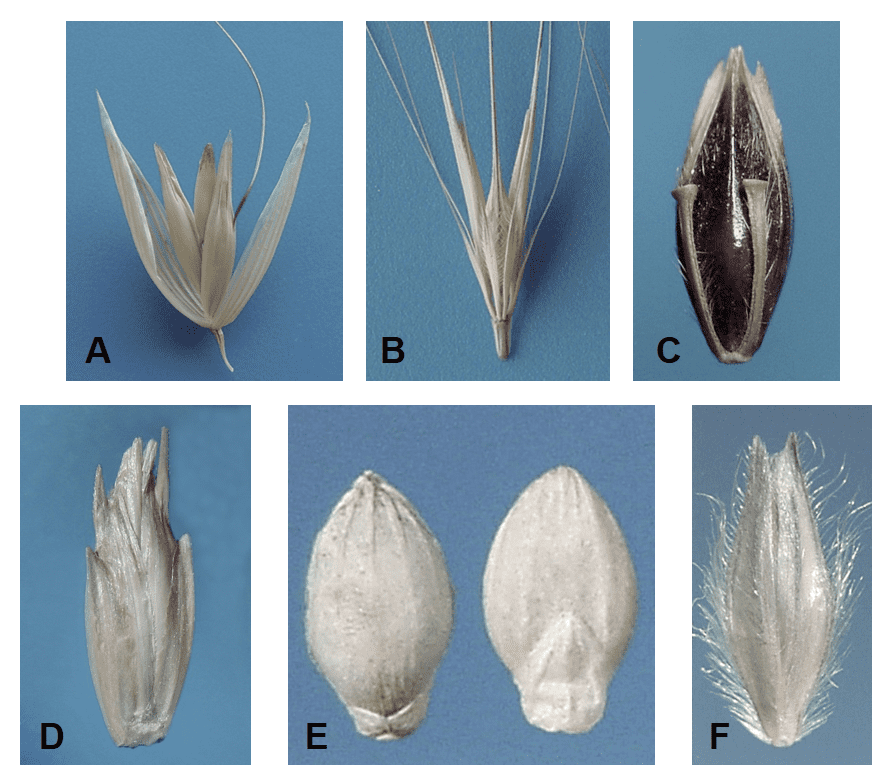
Figure 22. Examples of glume structural thickness and sheen: A – Avena sativa (oat) glumes are papery, dull, and glabrous; B – Hordeum murinum subsp. leporinum (wall barley) glumes are flat and narrow near base becoming long, stiff, and awn-like distally, they are also dull, and the margins are hairy in the lower one-third; C – Sorghum halepense (johnsongrass) glumes are leathery to shell-like, glossy, and hairy, particularly in the upper half; D – Triticum aestivum subsp. spelta (spelt) glumes are thick and leather-like; E – Setaria viridis (green bristlegrass) glumes are papery, dull, and glabrous; F – Alopecurus arundinaceus (creeping foxtail) glumes are papery, lustrous, and long pubescent.
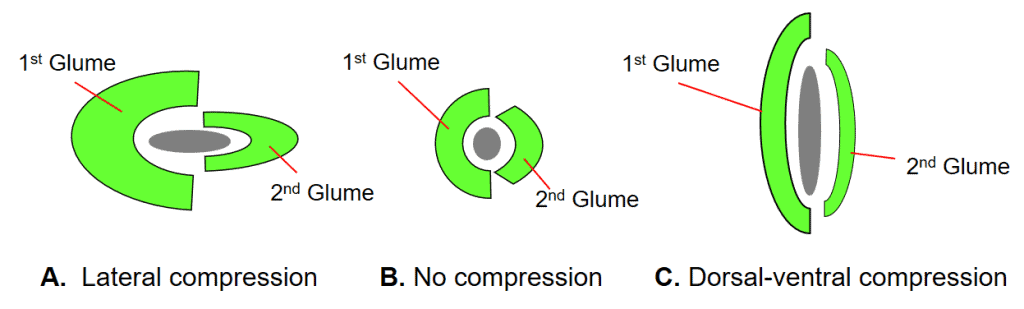
Figure 23. Spikelet compression types: A – Lateral compression, in which the first and second glume are folded inward longitudinally, and the two sides are compressed together to enclose the floret(s); B – No compression, in which the first and second glume encircle a nearly round floret(s); C – Dorsal-ventral compression, in which the first glume on the dorsal and second glume on the ventral sides of the spikelet, respectively, are compressed nearly flat towards each other enclosing the flattened floret(s). The green areas in the diagram represent the glumes and the grey areas represent the floret or group of florets.
Structural features of grass dispersal units can be found in Figures 24 through 42.
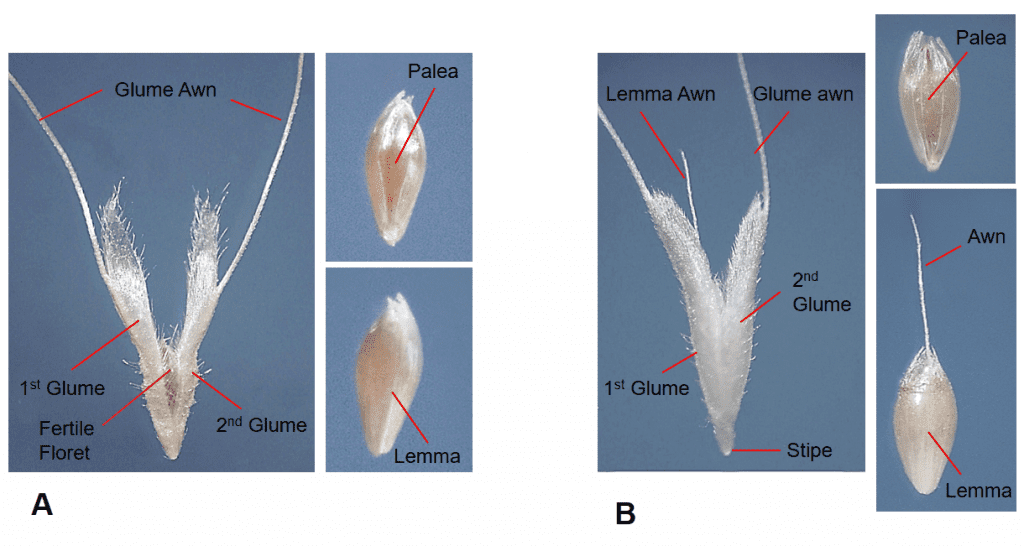
Figure 24. Spikelet compression types: A – Lateral compression, in which the first and second glume are folded inward longitudinally, and the two sides are compressed together to enclose the floret(s); B – No compression, in which the first and second glume encircle a nearly round floret(s); C – Dorsal-ventral compression, in which the first glume on the dorsal and second glume on the ventral sides of the spikelet, respectively, are compressed nearly flat towards each other enclosing the flattened floret(s). The green areas in the diagram represent the glumes and the grey areas represent the floret or group of florets.
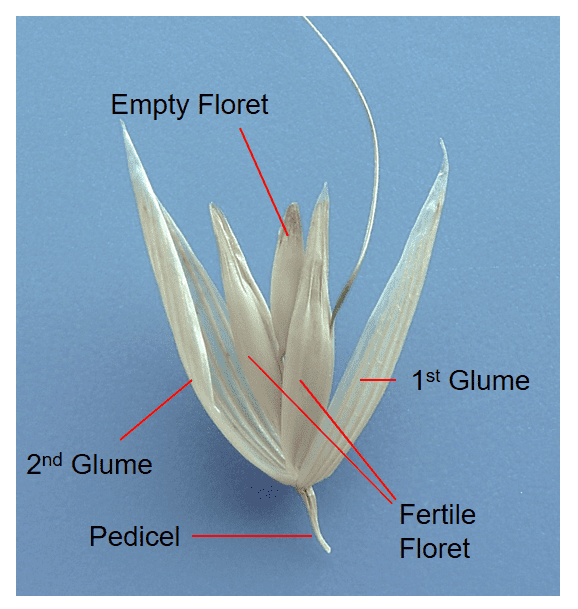
Figure 25.Avena sativa, oat, Subfamily Pooideae, Tribe Poeae, Subtribe Aveninae: the pedicellate spikelet has two glumes, one or more fertile florets, and the distal floret(s) may be sterile. The fertile lemmas may be awned slightly above the mid-point. In general, cultivated oats do not naturally disarticulate and are usually broken apart during post harvest conditioning. Wild species of Avena naturally disarticulate above the glumes and between the florets. The scars left from disarticulation at the base of the florets can be of diagnostic value for species identification. Individual florets and floret groups serve as dispersal units.
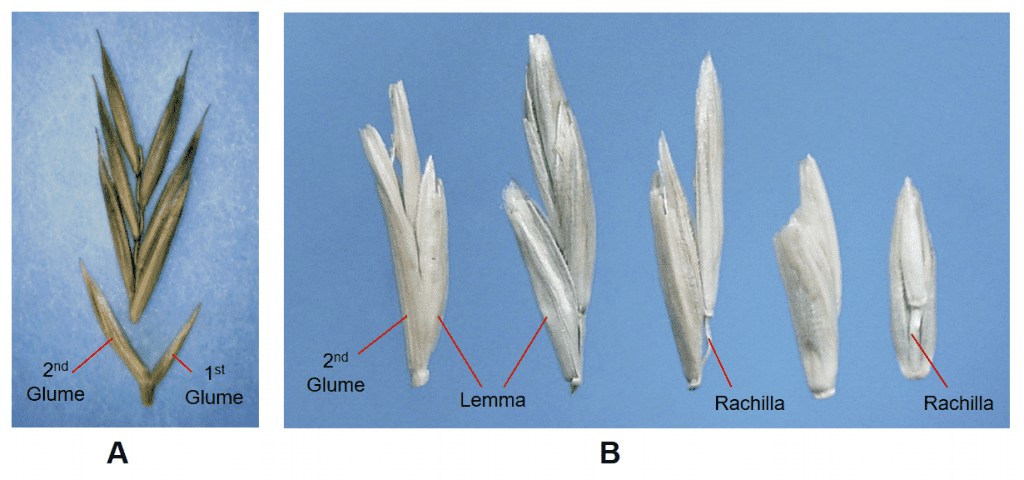
Figure 26. Examples of multiple floret spikelets in Poeae. A – Festuca rubra, red fescue, Subfamily Pooideae, Tribe Poeae, Subtribe Loliinae: Pedicellate spikelets with two glumes and several florets per spikelet (distal florets may be sterile) disarticulating above glumes and between florets at the rachilla nodes. B – Lolium perenne, perennial ryegrass, Tribe Poeae, Subtribe Loliinae: the first glume is lacking (absent) from all spikelets except the terminal spikelet of the inflorescence, the second glume is present, there are several florets per spikelet (distal florets may be sterile) disarticulating above glumes and between florets at the rachilla nodes. Dispersal units may consist of single florets, groups of florets remaining attached by the rachilla, or occasionally a spikelet (in commercial seed lots).
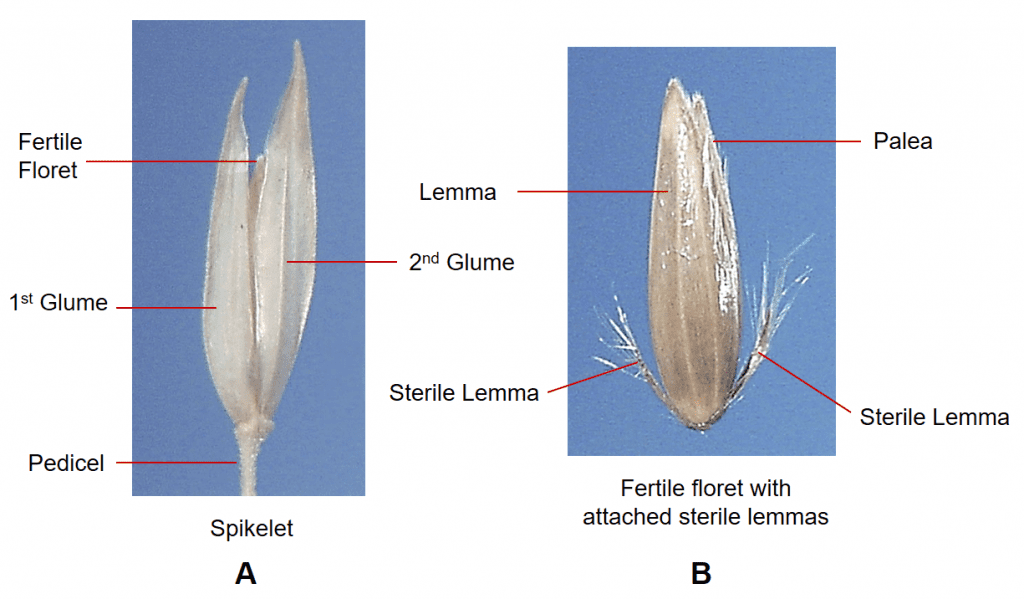
Figure 27. Phalaris arundinacea, reed canarygrass, Subfamily Pooideae, Tribe Poeae, Subtribe Phalaridinae: A – the pedicellate spikelet is laterally compressed and has two papery glumes; B – the spikelet contains one fertile floret subtended by two highly reduced sterile lemmas. Disarticulation occurs above glumes and the fertile floret with attached sterile florets serves as the dispersal unit.
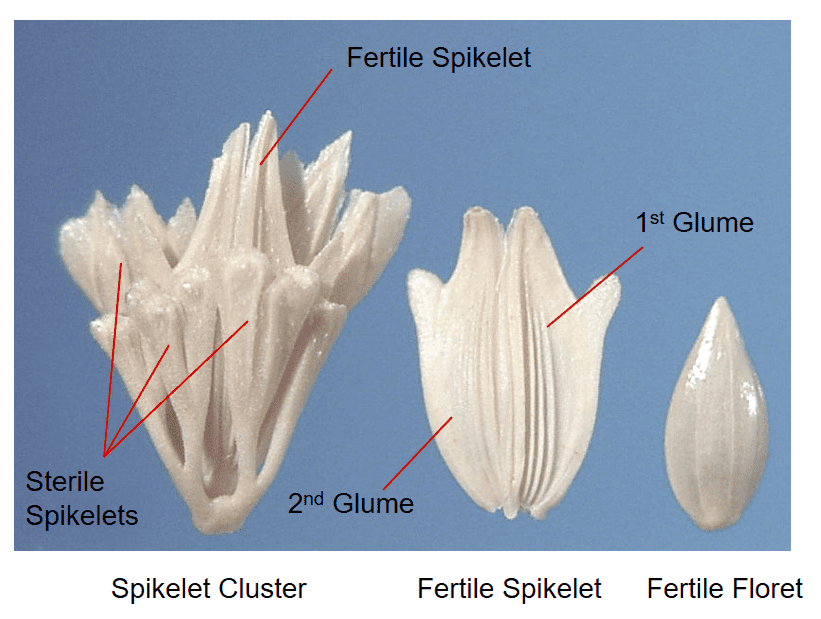
Figure 28. Phalaris paradoxa, hood canarygrass, Tribe Poeae, Subtribe Phalaridinae: Each fertile spikelet is surrounded by several sterile spikelets forming a cluster. The fertile spikelet and floret are laterally compressed. The glumes are papery, and the lemma is leathery to shell-like. Disarticulation from the plant typically occurs below the cluster of spikelets and the entire cluster serves as a dispersal unit. Individual fertile florets may also serve as dispersal units.
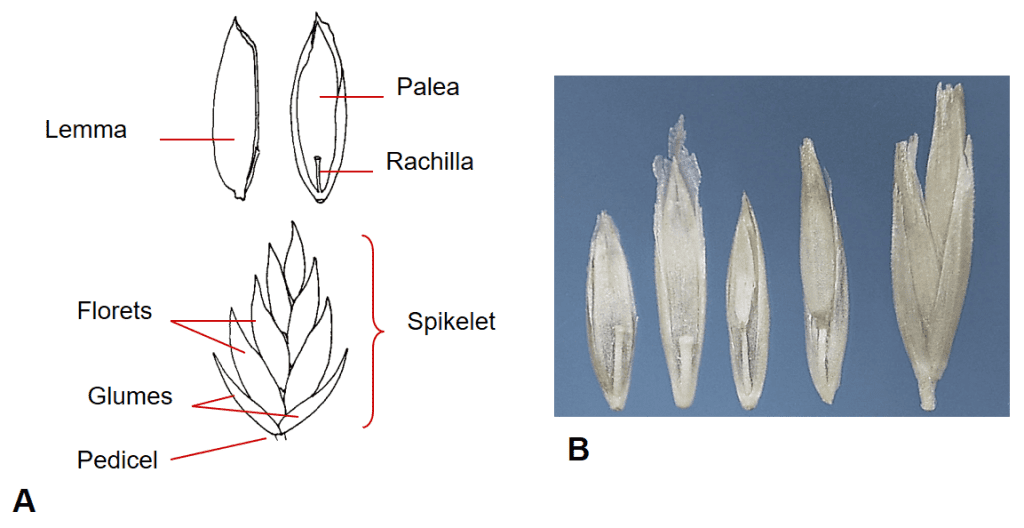
Figure 29. Poa spp., Subfamily Pooideae, Tribe Poeae, Subtribe Poinae: A – in Poa pratensis, Kentucky bluegrass, the multiple floret spikelet has two papery glumes, disarticulation usually occurs above glumes and between florets at the rachilla nodes; however, in commercial seed lots spikelets may occasionally remain intact. B – Poa secunda, big bluegrass, florets and spikelet (far right).
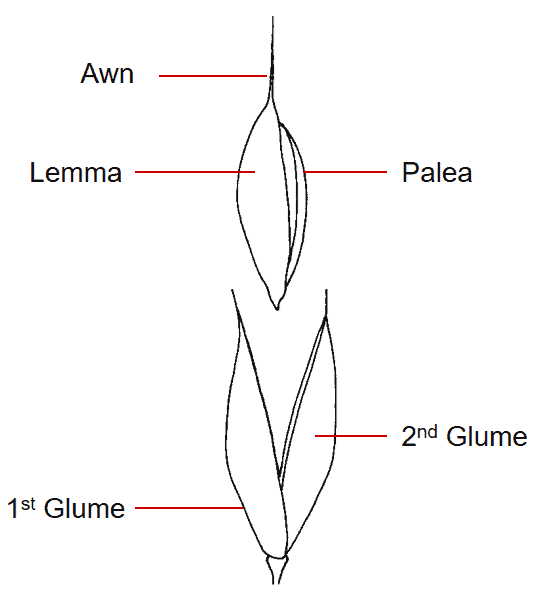
Figure 30. Oloptum miliaceum [Piptatherum miliaceum], smilo grass, Subfamily Pooideae, Tribe Stipeae: the pedicellate spikelet has two membranous glumes and one fertile floret. The lemma and palea are somewhat leathery, the lemma is awned from tip, the callus is pointed, and the disarticulation scar is round. Disarticulation occurs above glumes and the floret serves as the dispersal unit.
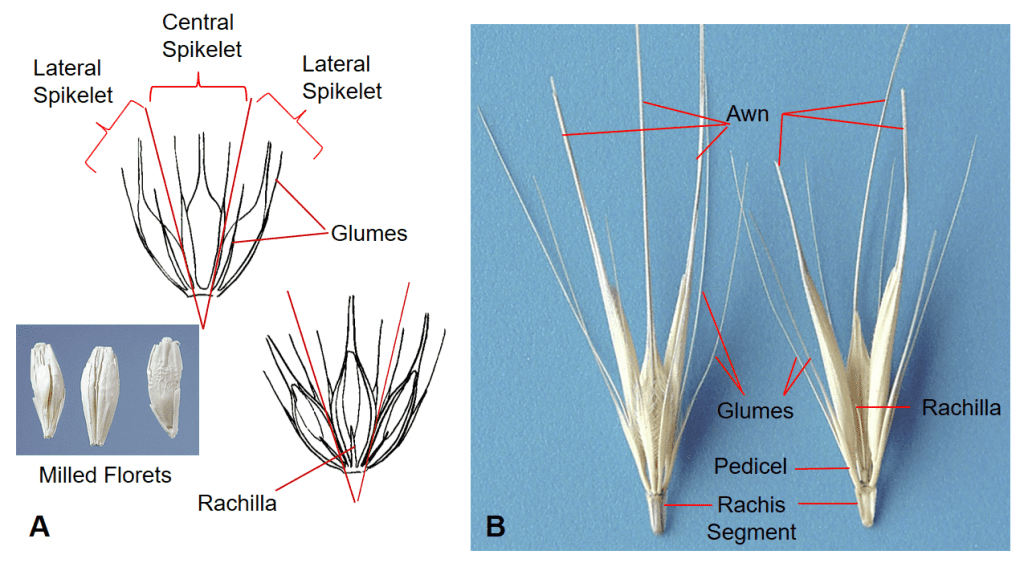
Figure 31. Hordeum spp., Subfamily Pooideae, Tribe Triticeae, Subtribe Hordeinae. A – Hordeum vulgare, barley, has three sessile, dorsal-ventrally compressed spikelets per rachis node, with two awn-like glumes flanking one awned floret per spikelet. Disarticulation occurs tardily at the rachis nodes in most cultivated varieties. Seed conditioning after harvest is used to break the florets apart for easy of mechanical planting. B – Hordeum murinum subsp. leporinum, wall barley, has three pedicellate, dorsal-ventrally compressed spikelets per rachis node, each spikelet has one awned floret and two glumes that are flattened near the base and extending into long awn-like structures. Disarticulation occurs at the rachis nodes and the three-spikelet group remains together as the dispersal unit.
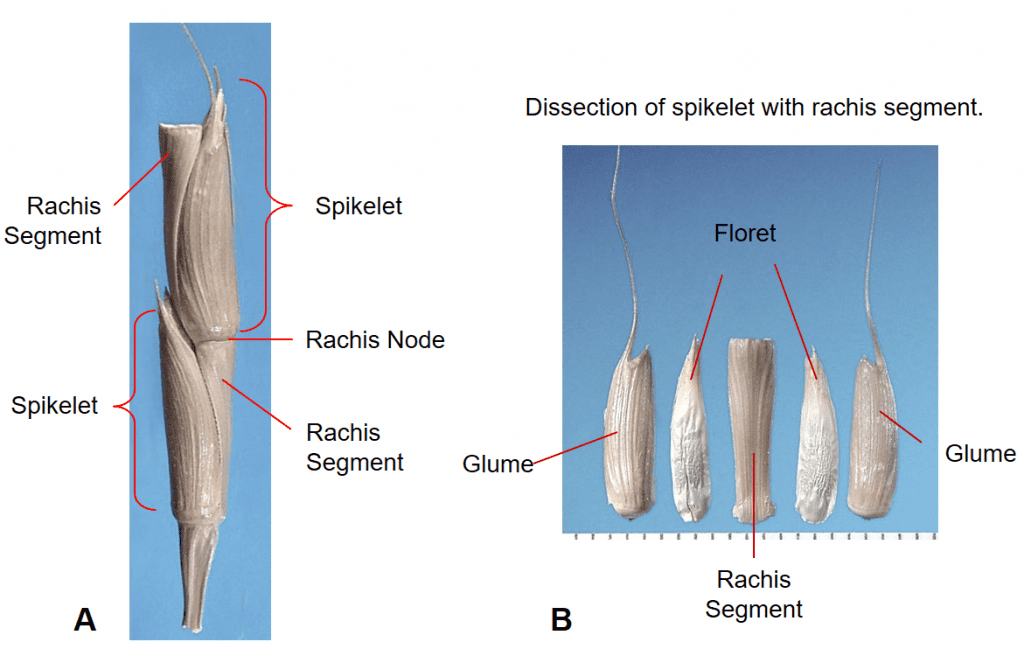
Figure 32. Aegilops cylindrica, goatgrass, Subfamily Pooideae, Tribe Triticeae, Subtribe Triticinae: the spike inflorescence is nearly cylindrical with one sessile spikelet per rachis node; each spikelet has two awned glumes that are thick and leathery and completely enclose one or more florets; disarticulation occurs at the rachis nodes and the spikelet with attached rachis segment becomes the dispersal unit. A – section of inflorescence with two spikelets. B – Dissected spikelet showing the components.
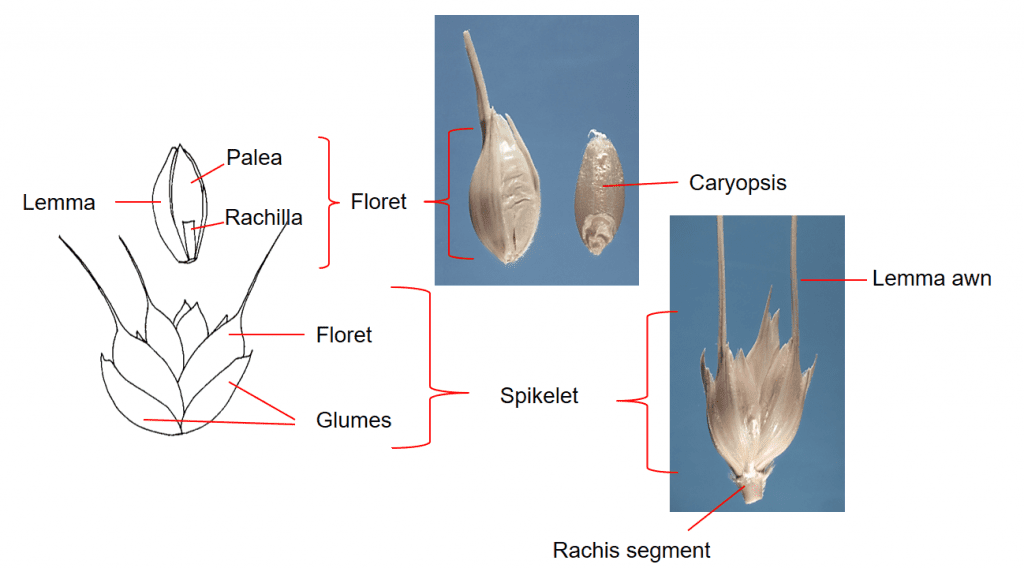
Figure 33. Triticum aestivum ssp. aestivum, common wheat, Subfamily Pooideae, Tribe Triticeae, Subtribe Triticinae: the spike inflorescence has one sessile spikelet per rachis node. Each spikelet has two glumes and three to nine florets per spikelet with up to five florets producing caryopses. Disarticulation of the inflorescence occurs at the rachis nodes in wild types of Triticum spp. and the spikelet is the dispersal unit. Cultivated varieties have been bred to not disarticulate at maturity. Caryopses in cultivated are usually removed via mechanical means.
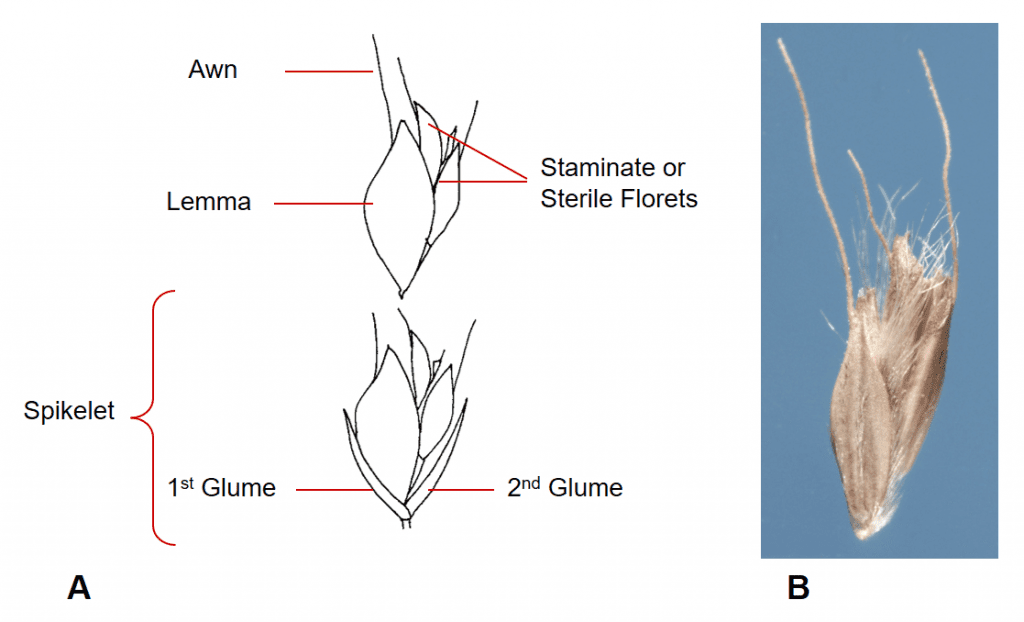
Figure 34. Chloris gayana, rhodesgrass, Subfamily Chloridoideae, Tribe Cynodonteae, Subtribe Eleusininae: A – spikelet has two glumes, a single fertile floret, and one or more staminate or sterile florets; B – disarticulation usually occurs above the glumes with the fertile and the staminate or sterile florets remaining attached to each other as the dispersing as a unit.
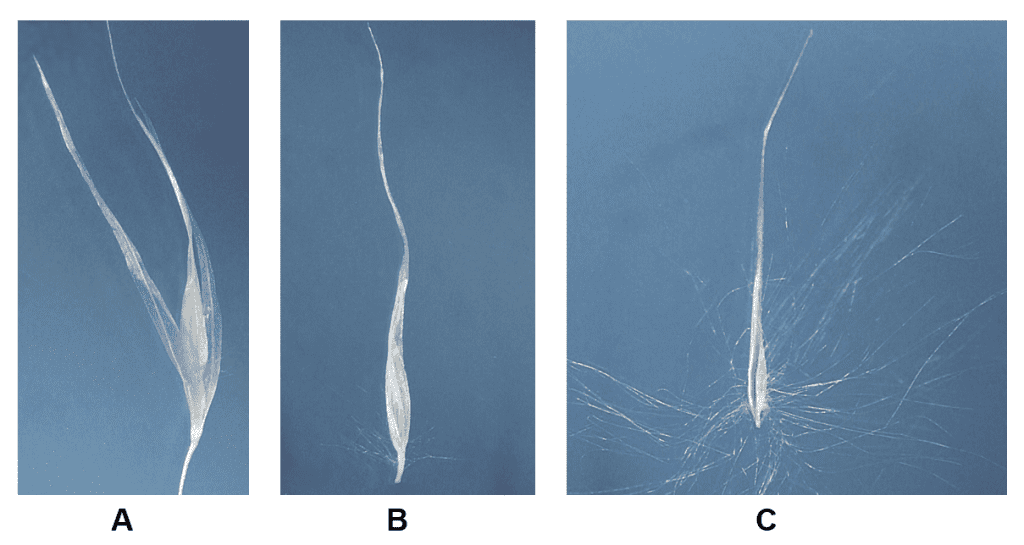
Figure 35. Cortaderia selloana, pampas grass, Subfamily Danthonioideae, Tribe Danthonieae: the plants are usually dioecious (staminate and pistillate inflorescences occurring on separate plants), occasionally monoecious staminate and pistillate inflorescences occurring on the same plant); spikelets have several florets, the glumes, lemmas, and paleas are hyaline; disarticulation occurs above the glumes and between the florets. A – Staminate spikelet. B – Staminate floret in which the lemma can be glabrous or sparsely pubescent near base and on the callus. C – Pistillate floret in which the lemma and callus are covered with long silky hairs and the lemma is extended into a long awn-like shape.
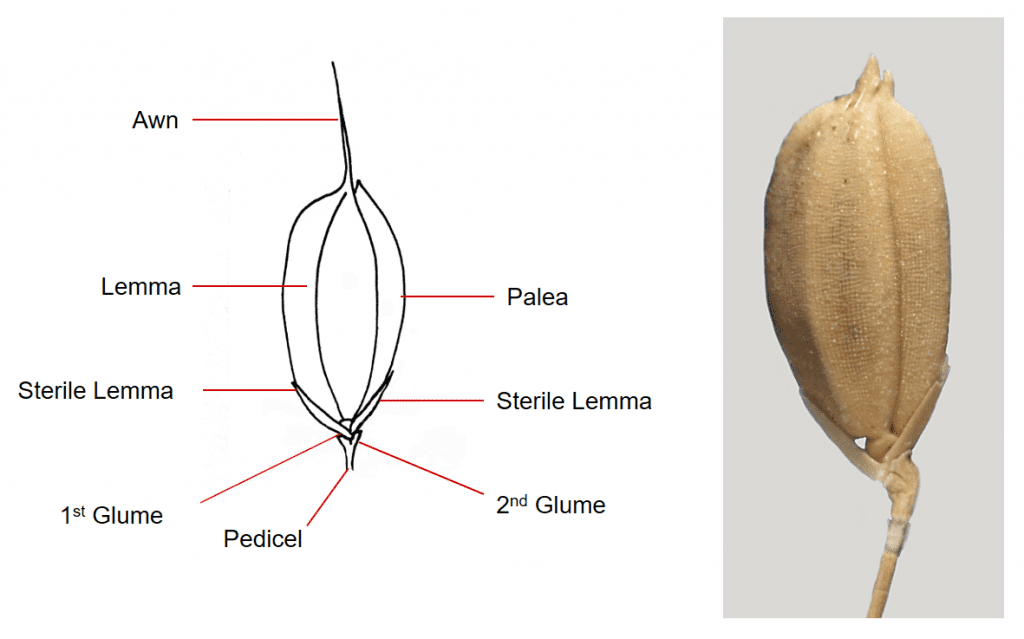
Figure 36. Oryza sativa, rice, Subfamily Oryzoideae, Tribe Oryzeae, Subtribe Oryzinae. The spikelet is pedicellate, laterally compressed, and the glumes highly reduced to a two lobed cup-like structure at tip of the pedicel. Two sterile lemmas are attached below the single fertile floret. The lemma and palea of the fertile floret are leathery to shell-like. Disarticulation occurs above the glumes and the dispersal unit consists of the fertile floret and two sterile lemmas.
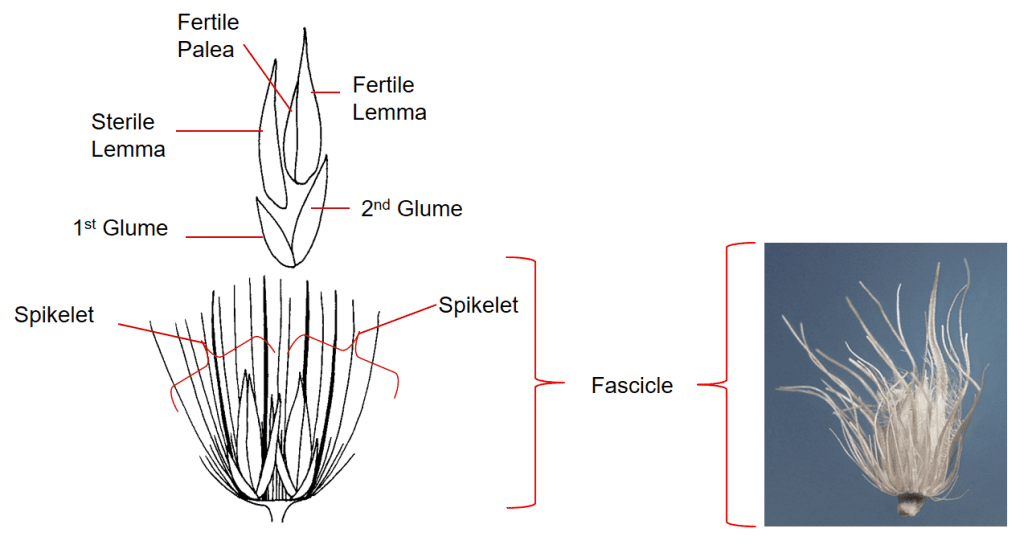
Figure 37. Cenchrus ciliaris [Pennisetum ciliare], hairy buffelgrass, Subfamily Panicoideae, Tribe Paniceae, Subtribe Cenchrinae: Numerous bristles subtend the spikelet groups forming a dispersal unit called a fascicle. The fascicle consists of 1-12 spikelets and the cluster of bristles of various lengths, some of which are fused and flattened for part of their length. The spikelets are sessile. Each spikelet has two glumes, the lower floret is staminate or sterile and the upper floret is fertile. Disarticulation occurs below the fascicle and the fascicle serves as the dispersal unit.
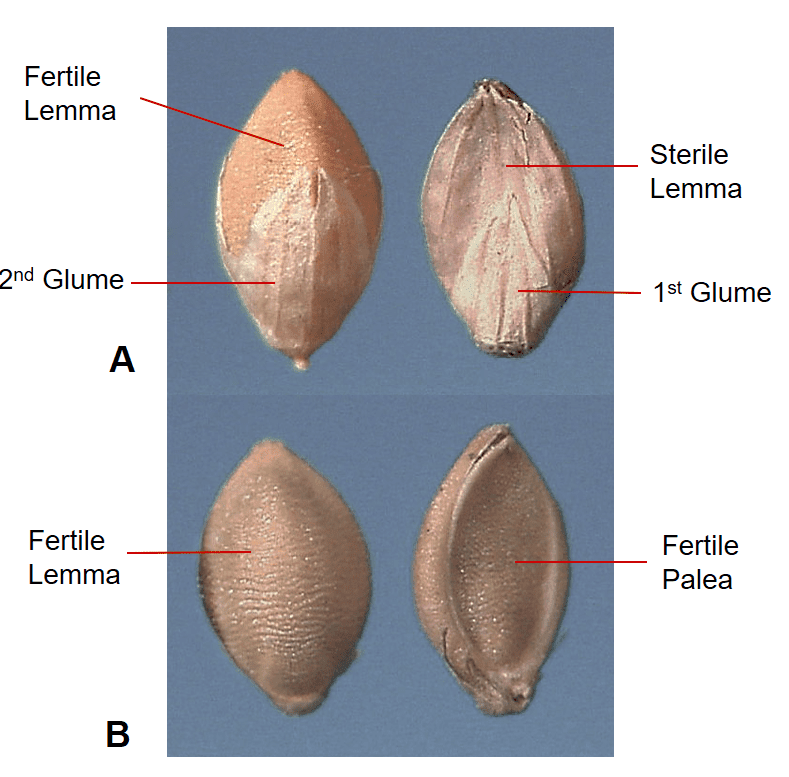
Figure 38. Setaria pumila subsp. pumila, yellow bristlegrass, Subfamily Panicoideae, Tribe Paniceae, Subtribe Cenchrinae: the spikelet has two papery glumes of unequal length and one papery sterile lemma attached below fertile floret (A), and one fertile floret with a shell-like lemma and palea (B); disarticulation occurs below the glumes and the spikelet is the dispersal unit.
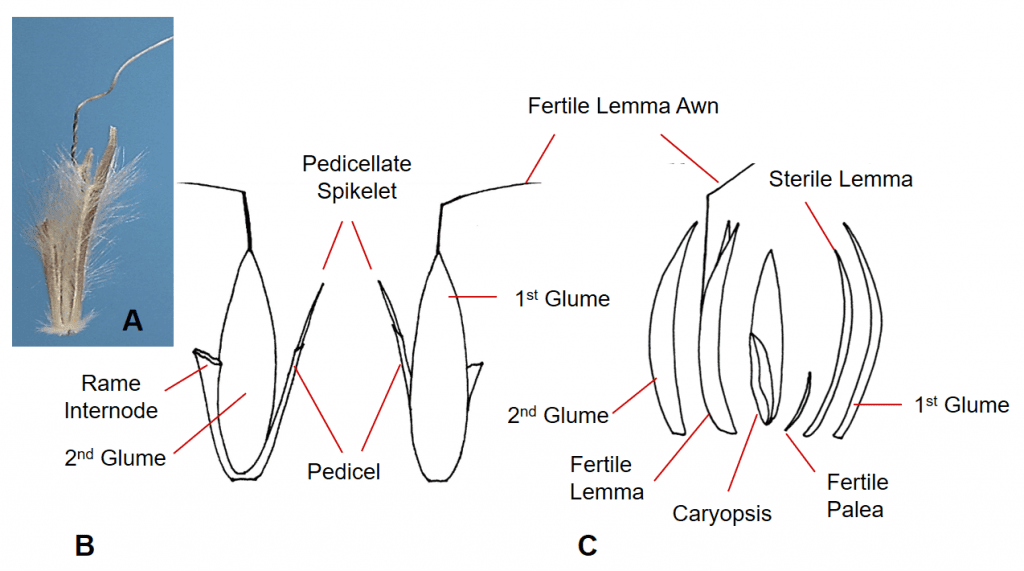
Figure 39. A – Schizachyrium scoparium, little bluestem, Subfamily Panicoideae, Tribe Andropogoneae, Subtribe Andropogoninae: A – the sessile spikelet has two shell-like glumes, one fertile floret with hyaline lemma and palea and a bent and twisted lemma awn, and one hyaline sterile lemma; the structures can be obscured by hairs covering many of the structures; disarticulation occurs below the glumes. B – The dispersal unit consists of a spikelet pair with one sessile fertile spikelet and one sterile or staminate pedicellate spikelet plus the rame internode that extends from the base of the sessile spikelet to the base of the next sessile spikelet. C – Dissected sessile spikelet showing the components.
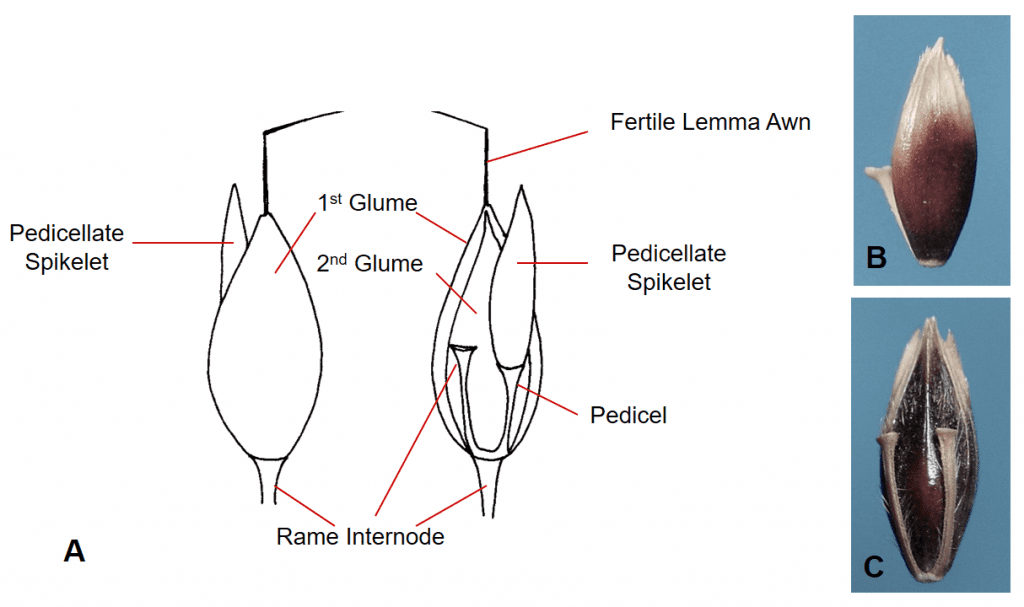
Figure 40. Sorghum halepense, Johnsongrass, Subfamily Panicoideae, Tribe Andropogoneae, Subtribe Andropogoninae: the sessile spikelet has two shell-like glumes, one hyaline sterile lemma, and one fertile floret with hyaline lemma and palea and a bent and twisted lemma awn; the first glume wraps around to conceal the margin of the second glume; disarticulation occurs below the glumes. A – The dispersal unit typically consists of a spikelet pair with one sessile fertile spikelet and one sterile or staminate pedicellate spikelet plus the rame internode that extends from the base of the sessile spikelet to the base of the next sessile spikelet; the pedicellate spikelet may or may not remain attached. B – First glume view of the sessile spikelet. C – Second glume view of the sessile spikelet showing the pedicel and rame internode attached to the second glume side of the dispersal unit.
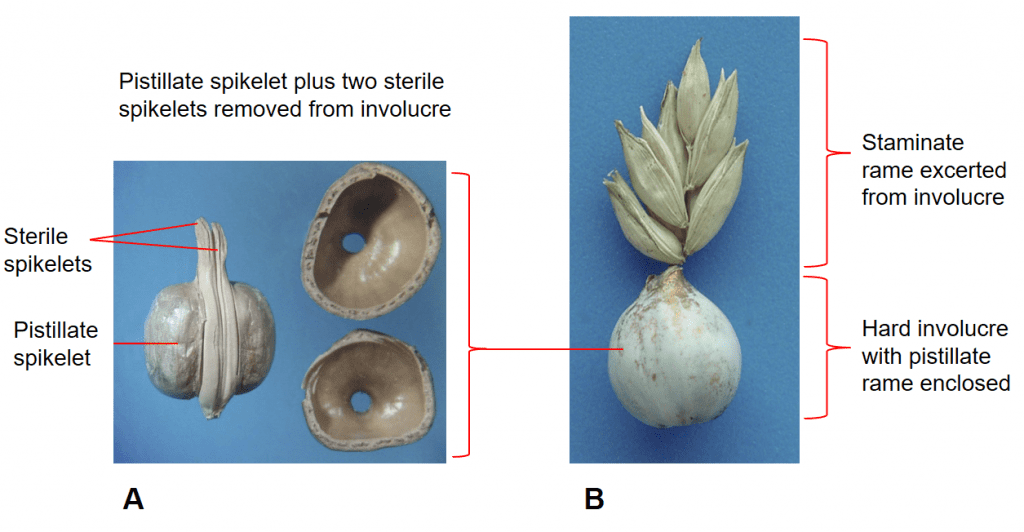
Figure 41. Coix lacryma-jobi, Job’s tears, Subfamily Panicoideae, Tribe Andropogoneae, Subtribe Coicinae: A – pistillate rame with one sessile pistillate spikelet and two pedicellate sterile spikelets enclosed within a hard involucre; B – staminate rame excerted from involucre, spikelets grouped in pairs or triplets with one being sessile and the others pedicellate.
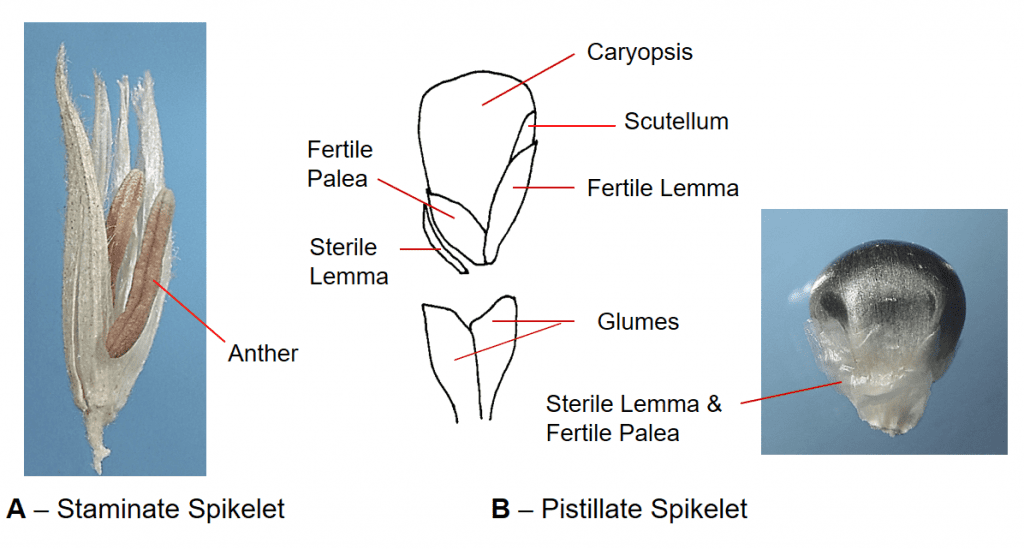
Figure 42. Zea mays, corn, Subfamily Panicoideae, Tribe Andropogoneae, Subtribe Tripsacinae. A – The staminate spikelets have two florets, each with three anthers. The staminate panicle inflorescence (also known as the tassel) is located at the top of the plant. B – The pistillate spikelets are sessile, each spikelet has a hyaline sterile floret and a fertile floret with hyaline lemma and palea. The spikelets are arranged in several rows along the central axis of the inflorescence. Disarticulation occurs above glumes but generally requires mechanical assistance. The pistillate inflorescence is located lower on the plant and is also known as the ear.
References
Meyer, D. J. L. 2020. Purity Testing Handbook. Contribution No. 41 to the Handbook on Seed Testing. Association of Official Seed Analysts. Washington, D.C. 250 pp.
Meyer, D. J. L. 2001. Basic botany for seed testing. Ch. 2. In: M. B. McDonald, T. Gutormson, and B. Turnipseed (eds.). Seed Technologist Training Manual. Society of Commercial Seed Technologists, Ithaca, NY.
Meyer, D. J. L. 2000. The Grass Caryopsis. California Department of Food & Agriculture.
Meyer, D. J. 1996. Understanding Grass Seed Units. AOSA News Letter 70(1):49-59. (Note: also re-published in the ISTA News Bulletin in 1996 or 1997.)
Terrell, E.E. 1971. Survey of occurrences of liquid or soft endosperm in grass genera. Bull. Torrey Bot. Club 98(5):264-268.
Photographs and illustrations by author unless otherwise noted.
Version 1.0
Copyright©2022 by Deborah J. Lionakis Meyer
All rights reserved: no part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, or otherwise, without the prior written permission of the author.



